Critical Process Disposable Media Filter Capsules
Critical Process Disposable Media Filter Capsules
SKU:PLE-b86473
High-performance Disposable Media Filter Capsules by Critical Process - FDA-compliant, USP-tested, ISO 9000 certified. Premium filtration for diverse applications.
Regular price
$89.99
Regular price
Sale price
$89.99
Unit price
per
Delivery via Maden
Expect your order to arrive on time.
Secure Payments
All orders are processed through a secure, PCI-compliant checkout.



The Critical Process Disposable Media Filter Capsules are meticulously engineered for efficient filtration needs, offering a diverse selection of pleated and depth media graded for general, food/beverage, electronic, and pharmaceutical applications. Compliant with FDA regulations, USP class 6 Toxicology tests, and ISO 9000 standards, these capsules minimize extractables, ensuring high-purity filtration for liquids and gases. Each capsule features a polypropylene membrane, heat-fused sealing, and a range of operational pressures and temperatures, validated through stringent testing processes. Ideal for industries requiring exceptional quality, reliability, and cost-effectiveness.
Product Specifications
Construction Materials Filtration Media
Polypropylene
Construction Materials Filtration Media Support Material
Polypropylene
Construction Materials Grade
Electronic
Construction Materials Housing
Polypropylene
Construction Materials Note
All materials of construction are FDA accepted. Final assemblies have been validated to pass USP class 6 Toxicology extractable tests, oxidizable substances for plastics, endotoxin level and other quality tests.
Construction Materials Sealing Method
Heat - Fused
Construction Materials Support Components Material
Polypropylene
Filtration Media 0
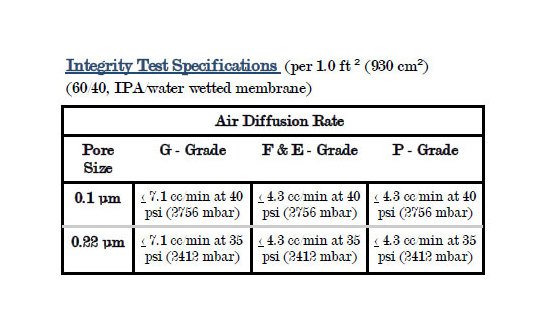
Polypropylene Membrane - PMPM Capsules are hydrophobic polypropylene membrane. They have excellent chemical compatibility and very low extractables. Widely used across the beverage, electronic and pharmaceutical industries. Applications include acids, bases, esters, solvents, alcohols, ketones, etchants and many others. Used in the filtration of compressed gas, fermentors and vent filters. Each cartridge module is individually diffusion tested using 60/40 IPA and water before it is released from manufacture. 100% integrity tested.
Flow Rate 0
See the Flow Rate table in more image which represents typical water flow at a one psi (69 mbar) pressure differential across a single 2 inch capsule 1.0 ft2 (930 cm2) of media with 1/2" FNPT ports. The test fluid is water at ambient temperature. Higher pressure drops is acceptable, but as flows increase the pressure drop of the housing becomes more apparent.
Maximum Operating Parameters Forward Differential Pressure At 20
50 psi | 3.4 bar
Maximum Operating Parameters Gases Operational Pressure At 20c
60 psi | 4.1 bar
Maximum Operating Parameters Liquid Operational Pressure At 20c
80 psi | 5.5 bar
Maximum Operating Parameters Operating Temperature At 30 Psi 14
110 ºF | 43 ºC
Maximum Operating Parameters Recommended Change Out Pressure
20 psid | 2.4 bar
Maximum Operating Parameters Reverse Differential Pressure At 20
40 psi | 2.7 bar
Pore Size Removal Ratings
0.03 to 100 µ
Quality Standards 0
Our goal is to ensure our customers the greatest possible value for their filtration dollar. We achieve both low cost manufacture and high quality by employing state of the art manufacturing equipment. This computer controlled equipment is highly automated, reducing hand operations that compromise quality. Each operation including assembly, testing, cleaning, drying and packaging is done in appropriately rated clean rooms. Critical Process Filtration manages an ISO 9000 facility that produces validated products to rigorous standards. Manufacturing is controlled using sophisticated MRP software that is networked to work stations in manufacturing centers and inspection points. During the manufacturing and inspection processes, data is collected âreal timeâ from machinery and measuring instruments. This allows variable and attribute data to be quickly and easily analyzed to facilitate constant improvements in both quality and cost.
Sanitization Sterilization Autoclave
250ºF (121ºC), 30 min, multiple cycles
Sanitization Sterilization Chemical Sanitization
Industry standard concentrations of hydrogen peroxide, paracetic acid, sodium hypochlorite and other selected chemicals. Sanitization protocols designed to extend the useful life of CP capsules are available from Critical Process Filtration, Inc.®.
Sanitization Sterilization Filtered Hot Water
194 ºF | 90 ºC
Sanitization Sterilization Inlet Outlet Options
1/2" Female NPT | 1/2" Male NPT | 1/4 Male NPT | 1/4" Female NPT | 3/8" Female NPT | Hose Barb | Sanitary
Sanitization Sterilization Pre Sterilized
Not Sterilized | Pre- Sterilized
Sanitization Sterilization Vent Drain
Standard with Luer- Lok¨ ports and caps
Specifications Capsule Length
2 in | 5 in | 10 in | 23 in | 30 in
Specifications Filter Mediamembrane
Polypropylene Membrane
Specifications Grade Definition
Designed to meet the special needs of the electronics and high purity chemical industries. To minimize extractables, each cartridge module is pulse, power flushed until the rinse effluent reaches 17+ megohm-cm and less than 3 ppb TOC. Each cartridge module is also individually tested.
Specifications Micron Rating
40.0 µ
Total Performance 0
Critical Process Filtration, Inc.® is a vertically integrated supplier of filtration products and services to industries in which filtration is considered to be a critical part of the manufacturing process. We manufacture a complete line of products to help you achieve all your filtration requirements from a single source.
Usp Biosafety 0
The materials used to construct the membrane capsule filters are non-toxic and meet the requirements for the MEM Elution Cytotoxicity Test and USP24 Plastic Class V1 121ºC Test.
Validation 0
P - grade capsules are validated using modified HIMA protocols at a challenge level of 107 organisms per cm2 of filter media.F & E - grade capsules are validated using modified HIMA protocols at a challenge level of 106 organisms per cm2 of filter media.G - grade capsules are validated using modified HIMA protocols at a challenge level of 104 organisms per cm2 of filter media.(0.22 µm challenged with Brevundimonas diminuta) (0.45 µm challenged with Serratia marscecens) (0.65 µm challenged with Saccharomyces cerevisiae).




