Critical Process Disposable Media Filter Capsules
Critical Process Disposable Media Filter Capsules
SKU:PLE-e224de
Nano Spun NSPD Disposable Media Capsules for Pharmaceutical Grade Filtration Needs
Regular price
$69.99
Regular price
Sale price
$69.99
Unit price
per
Delivery via Maden
Expect your order to arrive on time.
Secure Payments
All orders are processed through a secure, PCI-compliant checkout.



The Critical Process Disposable Media Filter Capsules are meticulously engineered to meet the stringent filtration requirements of the pharmaceutical industry. With a Nano Spun NSPD membrane and 3.0 µ micron rating, these capsules offer exceptional retention capabilities. Constructed with FDA-accepted polypropylene materials, these capsules are designed for maximum filtration efficiency and durability. Suitable for liquid and gas applications, these capsules undergo rigorous testing to ensure quality, traceability, and compliance with industry standards.
Product Specifications
Construction Materials Filtration Media
Polypropylene 99%
Construction Materials Filtration Media Support Material
Polypropylene
Construction Materials Grade
Pharmaceutical
Construction Materials Housing
Polypropylene
Construction Materials Note
All materials of construction are FDA accepted. Final assemblies have been validated to pass USP class 6 Toxicology extractable tests, oxidizable substances for plastics, endotoxin level and other quality tests.
Construction Materials Sealing Method
Heat - Fused
Construction Materials Support Components Material
Polypropylene
Filtration Media 0
Nano-Spun - NSNSPD Media filter cartridges have been designed to hold large amounts of contaminant and still provide 99% retention efficiencies at the rated pore size. Made of 100% polypropylene these cartridges can be used wherever a high contamination load application requires retention efficiencies above nominal. NS is an excellent choice where protection of more expensive membrane filters is required or a high efficiency depth filter is all that is required.
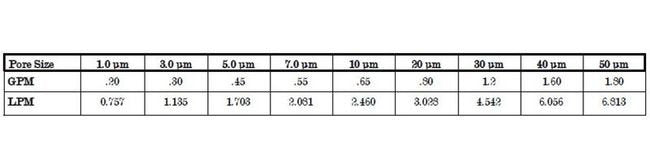
Flow Rate 0
See the Flow Rate table in more image which represents typical water flow at a one psi (69 mbar) pressure differential across a single 2 inch capsule 1.0 ft2 (5.08 cm) of media with 1/2 inch FNPT ports. The test fluid is water at ambient temperature. Higher pressure drops is acceptable, but as flows increase the pressure drop of the housing becomes more apparent.
Maximum Operating Parameters Forward Differential Pressure At 20
50 psi | 3.4 bar
Maximum Operating Parameters Gases Operational Pressure At 20c
60 psi | 4.1 bar
Maximum Operating Parameters Liquid Operational Pressure At 20c
80 psi | 5.5 bar
Maximum Operating Parameters Operating Temperature At 30 Psi 14
110 ºF | 43 ºC
Maximum Operating Parameters Recommended Change Out Pressure
20 psid | 2.4 bar
Maximum Operating Parameters Reverse Differential Pressure At 20
40 psi | 2.7 bar
Pore Size Removal Ratings
0.03 to 100 µ
Quality Standards 0
Our goal is to ensure our customers the greatest possible value for their filtration dollar. We achieve both low cost manufacture and high quality by employing state of the art manufacturing equipment. This computer controlled equipment is highly automated, reducing hand operations that compromise quality. Each operation including assembly, testing, cleaning, drying and packaging is done in appropriately rated clean rooms. Critical Process Filtration manages an ISO 9000 facility that produces validated products to rigorous standards. Manufacturing is controlled using sophisticated MRP software that is networked to work stations in manufacturing centers and inspection points. During the manufacturing and inspection processes, data is collected âreal timeâ from machinery and measuring instruments. This allows variable and attribute data to be quickly and easily analyzed to facilitate constant improvements in both quality and cost.
Sanitization Sterilization Autoclave
250ºF (121ºC), 30 min, multiple cycles
Sanitization Sterilization Chemical Sanitization
Industry standard concentrations of hydrogen peroxide, paracetic acid, sodium hypochlorite and other selected chemicals. Sanitization protocols designed to extend the useful life of CP capsules are available from Critical Process Filtration, Inc.®.
Sanitization Sterilization Filtered Hot Water
194 ºF | 90 ºC
Sanitization Sterilization Inlet Outlet Options
1/2" Female NPT | 1/2" Male NPT | 1/4 Male NPT | 1/4" Female NPT | 3/8" Female NPT | Hose Barb | Sanitary
Sanitization Sterilization Pre Sterilized
Not Sterilized | Pre- Sterilized
Sanitization Sterilization Vent Drain
Standard with Luer- Lok¨ ports and caps
Specifications Capsule Length
2 in | 5 in | 10 in | 23 in | 30 in
Specifications Filter Mediamembrane
Nano Spun NSPD
Specifications Flow Rate
0.30 gpm | 1.135 lpm
Specifications Grade Definition
The media utilized in these capsules is optimized for retention and durability to meet the extra security needs of the pharmaceutical industry. Each module is rinsed with 17+ megohm-cm water to remove potential extraneous manufacturing debris. Each cartridge module is individually tested for integrity. Further, representative cartridge modules have been validated to show equivalent retention characteristics per the deigned protocol.
Specifications Micron Rating
3.0 µ
Total Performance 0
Critical Process Filtration, Inc.® is a vertically integrated supplier of filtration products and services to industries in which filtration is considered to be a critical part of the manufacturing process. We manufacture a complete line of products to help you achieve all your filtration requirements from a single source.
Usp Biosafety 0
The materials used to construct the membrane capsule filters are non-toxic and meet the requirements for the MEM Elution Cytotoxicity Test and USP24 Plastic Class V1 121ºC Test.




