D60 Cured Durometer Hardness Medical Wearable Adhesive
D60 Cured Durometer Hardness Medical Wearable Adhesive
SKU:MED-11a2c2
Cutting-edge D60 Cured Durometer Hardness Medical Wearable Adhesive with UV/LED cure, biocompatibility, and ISO compliance for medical device assembly.
Regular price
$29.99
Regular price
Sale price
$29.99
Unit price
per
Delivery via Maden
Expect your order to arrive on time.
Secure Payments
All orders are processed through a secure, PCI-compliant checkout.
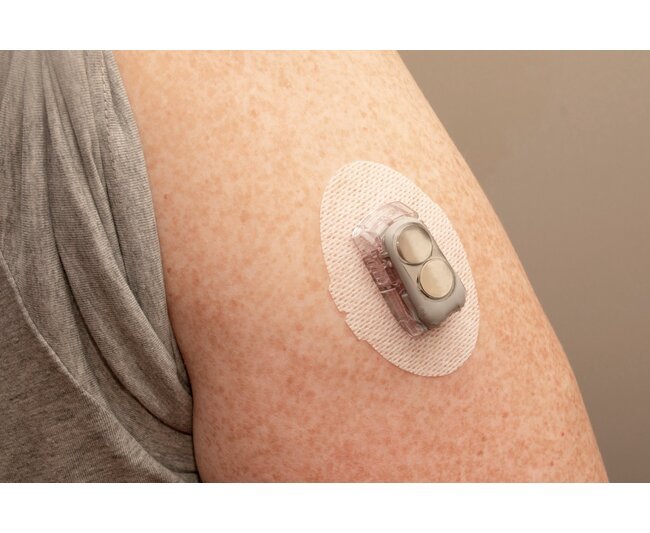
The D60 Cured Durometer Hardness Medical Wearable Adhesive from Dymax is a cutting-edge solution for assembling medical wearables with its UV/LED light curable formula. This adhesive is free of skin irritants like IBOA and TPO, meeting ISO 10993-10 / -23 standards for sensitization and irritation, as well as ISO 10993-5 for cytotoxicity. With superior adhesion to substrates like PC, PVC, SS, PI, TPU, and more, this biocompatible adhesive is perfect for sealing, encapsulating, coating, and bonding components in medical devices. Its low water absorption and quick curing properties make it ideal for prolonged skin contact, ensuring reliability and safety in wearable medical applications.
Product Specifications
Additional Information Additional Information
Low Water Absorption Encapsulant, Coating and Bonding Adhesive for Wearable Medical DevicesDymax® 2022-MW is a versatile general bonding adhesive, coating, and encapsulant with low water absorption and minimized water ingression. It offers extremely low water absorption of 0.5% making it ideal for the assembly or encapsulation of electronic wearable medical device components.Formulated without IBOA, a known skin irritant, and TPO, a material of concern, 2022-MW is compliant with ISO 10993-5 for cytotoxicity standards. This classification makes it an ideal material for wearable medical devices where proximity to the skin for extended periods of time is a concern.2022-MW is recommended for use as a coating or encapsulant on RFID chips or sensors housing assemblies and single- or multi-use medical devices where moisture ingression is a concern. It bonds well to stainless steel, aluminum, glass, and printed circuit boards.This material has no solvents added and cures in seconds upon exposure to broad-spectrum UV or 365 nm LED light.
Features Features
Low water absorption: 0.5%Biocompatible 10993-5 cytotoxicity standardsIBOA Free (designed without isobornyl acrylate)UV/Broad Spectrum and LED curable at 365nmSuperior adhesion to a wide range of substratesNo solvents added
Specifications Cured Durometer Hardness
D60
Specifications Cured Elongation At Break
8 %
Specifications Nominal Viscosity At 20 Revolutions Per Minute Rp
700 cP
Specifications Recommended Substrates
Acrylonitrile Butadiene Styrene (ABS) | Aluminum | Glass | Polycarbonate (PC) | Polyethylene Terephthalate Glycol (PETG) | Polymide (PI) | Polyurethane (PU) | Stainless Steel (SS) | Styrene-Acrylonitrile (SAN)

